I've been soliciting advice from diverse professional bloggers about making this blogging thing into a vocation, and there was a solid consensus that while wonky political types are fairly common, science writers are much less so. What's more, taking a look at the science blogosphere, the majority are either biologists or physicists. So, I'm starting a new series today on chemistry!
Now, I know what you're thinking: chemistry sucks! Not so. It's true, chemistry does seem to have the worst reputation of the hard sciences. My favorite sub-discipline, organic chemistry, is usually considered a form of madness. (Damn pre-meds.) I'm here to tell you that, while it might not have the sexiness of astronomy or the interesting squishiness of biology, chemistry can be fun and fascinating. Onward!
The first topic today is the line diagram (or skeletal formula). This is a simple chemical shorthand that lazy organic chemists use to avoid having to draw endless carbons and hydrogens. We'll need it to understand future pictures. There are basically four rules: 1) lines represent bonds, 2) every vertex or terminus is a carbon, 3) all carbons must have four bonds, and 4) hydrogens bonded to carbon are omitted:
Here we've got two pictures of ethanol. On the left is a simple arrangement diagram, showing which atoms are connected to each other. On the right is the line diagram of the same molecule. The line ends on the left (making a terminus), so that counts as a carbon, and the angle (vertex) in the middle counts as a carbon. The left carbon has only one bond shown explicitly, but by rule 3 all carbons must have four bonds, so by rule 4 we fill out the remaining bonds with hydrogens. Thus, like the left picture, there are three hydrogens bonded to the left carbon. By a similar argument, the middle carbon is bonded to two implied hydrogens. Still with me? (See here for more.)
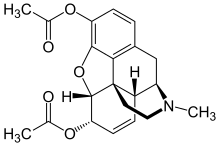
This is heroin. You might notice that it's slightly more complex than ethanol. Don't be afraid, it's mostly just a lot bigger. The parallel lines up there represent double bonds, where two pairs of electrons are being shared between the atoms instead of one. (That means that any carbon with a double bond is only bonded to three other atoms instead of four.) Other than that the only issue is the bolded and dashed bond lines; these are meant to represent orientation. The bold ones are coming up out of your computer screen at you, while the hashed ones like the oxygen on the bottom (O = oxygen) are going down into the screen.
So why is heroin not actually a drug? Let's compare heroin and morphine:
Fairly similar, eh? In fact, they're exactly the same except for those funny groups on the left side of the heroin (there's one attached to our friend the bottom oxygen). Where morphine has an -OH (oxygen-hydrogen) group, or an alcohol, heroin has an acetyl group. Now, "acetyl" looks scary, but it's actually very close to something I'm sure you're familiar with: vinegar.
This is acetic acid, the molecule that gives vinegar its smell, taste, and acidic properties. If you look closely, you can see that all that differentiates heroin from morphine is two of those bad boys stuck on the side. If you took some heroin, your body would go to work on it by putting it through hydrolysis, busting off those acetyl groups and converting it to morphine. Therefore, heroin is a prodrug, meaning that it's an inactive form of another drug that gets metabolized in the body to the active form. When you take heroin, the thing that eventually ends up in your brain's receptor sites is morphine.
So why does anyone take heroin? The thing about morphine is that it's a big, greasy molecule, and has some trouble crossing the blood-brain barrier. Those acetyl groups, though, make heroin a lot more fat soluble, enabling it to sneak into the brain a lot more easily—effectively making it three times more potent than morphine, but only if injected. If you eat heroin, the first round of metabolism in your digestive tract converts most of it to morphine before it can make it to your brain. You've got to bypass that by going straight into the bloodstream to get the extra potency.
That's it for today. Tips, comments, or suggestions are always welcome.
Now, I know what you're thinking: chemistry sucks! Not so. It's true, chemistry does seem to have the worst reputation of the hard sciences. My favorite sub-discipline, organic chemistry, is usually considered a form of madness. (Damn pre-meds.) I'm here to tell you that, while it might not have the sexiness of astronomy or the interesting squishiness of biology, chemistry can be fun and fascinating. Onward!
The first topic today is the line diagram (or skeletal formula). This is a simple chemical shorthand that lazy organic chemists use to avoid having to draw endless carbons and hydrogens. We'll need it to understand future pictures. There are basically four rules: 1) lines represent bonds, 2) every vertex or terminus is a carbon, 3) all carbons must have four bonds, and 4) hydrogens bonded to carbon are omitted:
Here we've got two pictures of ethanol. On the left is a simple arrangement diagram, showing which atoms are connected to each other. On the right is the line diagram of the same molecule. The line ends on the left (making a terminus), so that counts as a carbon, and the angle (vertex) in the middle counts as a carbon. The left carbon has only one bond shown explicitly, but by rule 3 all carbons must have four bonds, so by rule 4 we fill out the remaining bonds with hydrogens. Thus, like the left picture, there are three hydrogens bonded to the left carbon. By a similar argument, the middle carbon is bonded to two implied hydrogens. Still with me? (See here for more.)
 |
| Heroin |
This is heroin. You might notice that it's slightly more complex than ethanol. Don't be afraid, it's mostly just a lot bigger. The parallel lines up there represent double bonds, where two pairs of electrons are being shared between the atoms instead of one. (That means that any carbon with a double bond is only bonded to three other atoms instead of four.) Other than that the only issue is the bolded and dashed bond lines; these are meant to represent orientation. The bold ones are coming up out of your computer screen at you, while the hashed ones like the oxygen on the bottom (O = oxygen) are going down into the screen.
So why is heroin not actually a drug? Let's compare heroin and morphine:
  |
| Heroin (left) and morphine (right) |
Fairly similar, eh? In fact, they're exactly the same except for those funny groups on the left side of the heroin (there's one attached to our friend the bottom oxygen). Where morphine has an -OH (oxygen-hydrogen) group, or an alcohol, heroin has an acetyl group. Now, "acetyl" looks scary, but it's actually very close to something I'm sure you're familiar with: vinegar.
 |
| Acetic acid |
This is acetic acid, the molecule that gives vinegar its smell, taste, and acidic properties. If you look closely, you can see that all that differentiates heroin from morphine is two of those bad boys stuck on the side. If you took some heroin, your body would go to work on it by putting it through hydrolysis, busting off those acetyl groups and converting it to morphine. Therefore, heroin is a prodrug, meaning that it's an inactive form of another drug that gets metabolized in the body to the active form. When you take heroin, the thing that eventually ends up in your brain's receptor sites is morphine.
So why does anyone take heroin? The thing about morphine is that it's a big, greasy molecule, and has some trouble crossing the blood-brain barrier. Those acetyl groups, though, make heroin a lot more fat soluble, enabling it to sneak into the brain a lot more easily—effectively making it three times more potent than morphine, but only if injected. If you eat heroin, the first round of metabolism in your digestive tract converts most of it to morphine before it can make it to your brain. You've got to bypass that by going straight into the bloodstream to get the extra potency.
That's it for today. Tips, comments, or suggestions are always welcome.



Are these pictures to scale?
ReplyDeleteAh, not particularly. Big molecules tend to get all bendy, and there's a bit of distortion to represent them on a flat page. You mean between the heroin and the morphine, or just generally?
ReplyDeleteI'm not a chemist but I think actual molecules are a little smaller than those pictures. Just joking around.
ReplyDeleteIt's interesting to consider two opiates in terms of their actual chemical properties. Strange that the added acetic value in heroin actual makes it easier to enter the brain quickly. I would have assumed that morphine was the larger, clunkier molecule.
DOH! Well, I can see I'm living up to the chemist humorless pedant standard.
ReplyDeleteSize is important for drug behavior (ethanol is so tiny it sails right through the blood-brain barrier), but in this case it's the lack of O-H bonds in heroin that makes it more slippery. O-H bonds mean hydrogen bonding, about the most powerful form of intramolecular bonding (between separate molecules) around, and also good solubility in water. No hydrogen bonds means worse solubility in water, but better solubility in fats, which is the key for getting straight into the brain.
I would love to see more of these blog posts, Ryan. It's not that your political rants aren't welcome (I quite enjoy them, as they're both informative and funny), but it'd be awesome to see some science blogging. You're absolutely right - biology and physics absolutely dominate the science blogosphere, and we need some more chemistry representation.
ReplyDeleteOn a (slightly related) tangent, have you seen ToC ROFL yet? It's a tumblr (http://tocrofl.tumblr.com/) that aggregates the papers with the most ridiculous figures. I'm amazed at how many of these got (unironically) published...
-Dan UncomprehensibleLastNameOrfer